Inherited retinal diseases (IRDs) represent a diverse and complex group of genetic disorders affecting the retina—the light-sensitive layer of tissue at the back of the eye. Within the IRD umbrella are primary photoreceptor diseases, which specifically cause photoreceptor degradation or dysfunction. These cells are crucial for vision, and their loss of function leads to significant visual impairments.
BlueRock Therapeutics’ lead ophthalmology program is focused on primary photoreceptor diseases, including retinitis pigmentosa (RP) and cone-rod dystrophies.
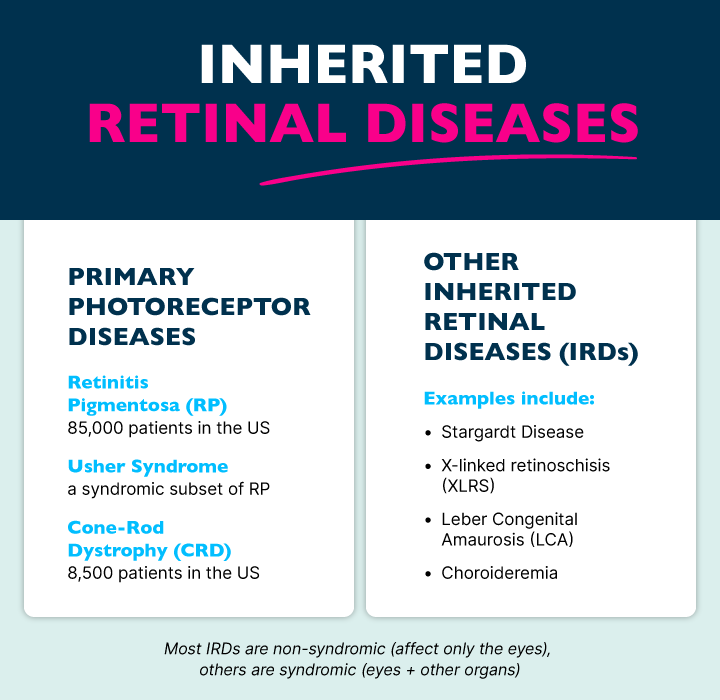
What are Primary Photoreceptor Diseases?
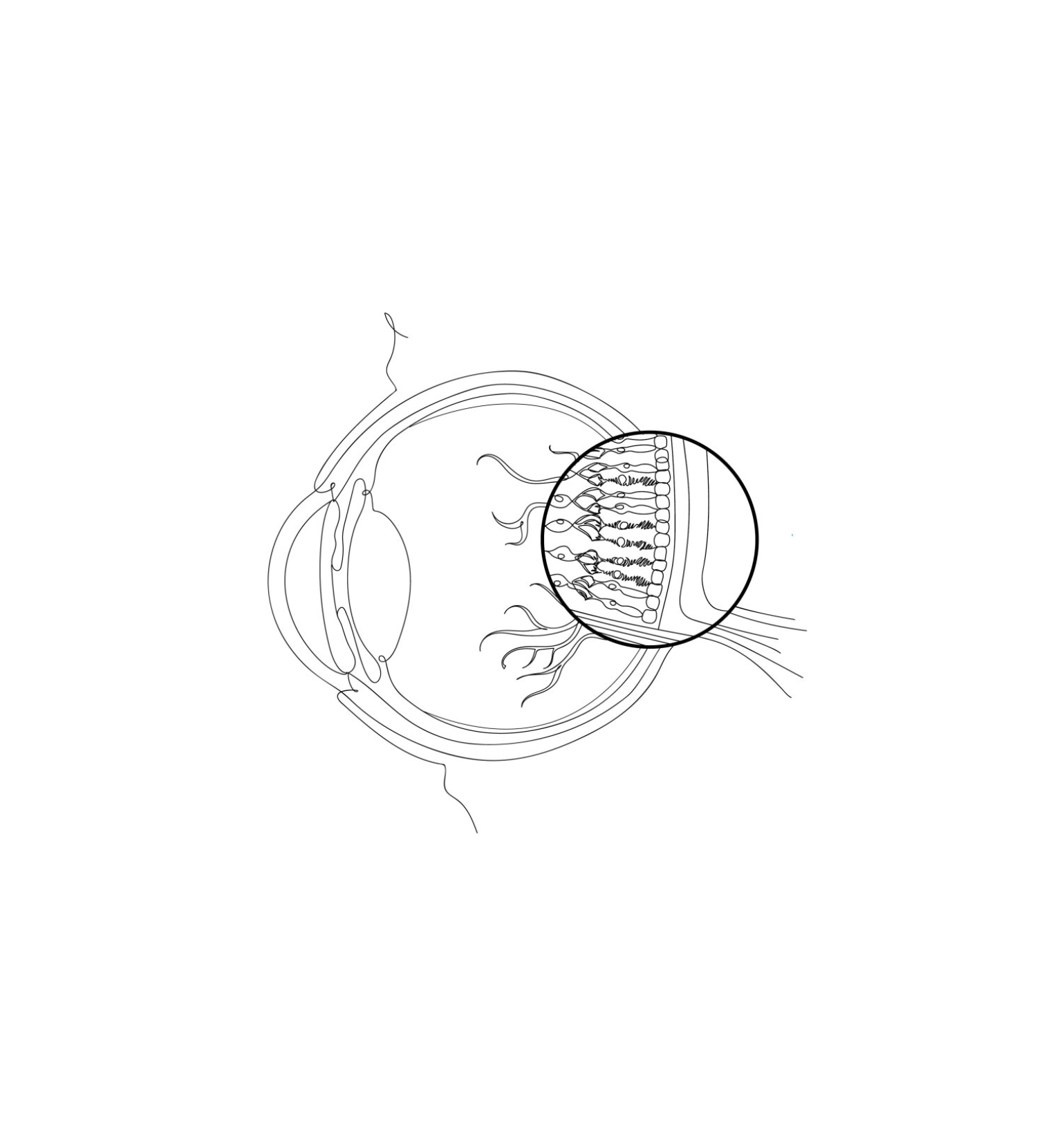
Primary photoreceptor diseases are driven by genetic mutations that cause the initial loss of function of photoreceptor cells, which are essential for converting light into visual signals. These cells have two distinct types—rods and cones. Rod cells are largely responsible for night and peripheral vision, while cone cells help with day vision, color perception, and visual clarity. These diseases are further characterized as rod-cone dystrophy or cone-rod dystrophy, depending on which cell type is affected first. Primary photoreceptor diseases affect an estimated 110,000 people in the United States (1 in ~3,000 for RP and 1 in ~30,000 for cone rod).
The Patient Experience and Current Treatment Landscape
Patients may experience varying symptoms, onset timings, and disease progression depending on the specific type of dystrophy they have. For instance, in rod-cone dystrophies like RP, patients often will notice a loss of night and peripheral vision first. The initial onset of vision loss greatly varies depending on the specific gene affected. For example, X-linked RP, which is a more severe form, might start to manifest in the teenage years, while autosomal dominant RP might not show symptoms until the fourth or fifth decade of life. In contrast, cone-rod dystrophies typically present in childhood, where patients often have extreme light sensitivity or develop photophobia. Almost all patients with this subtype become legally blind by adulthood.
The journey to a definitive diagnosis can be challenging, often requiring visits with multiple ophthalmologists before an eventual referral to an inherited retinal disease expert and/or genetic specialist. Treatment options are also limited. Currently there is only one FDA-approved gene therapy, which addresses a specific mutation associated with Leber congenital amaurosis. This therapy can partially restore vision, but it is not applicable to the majority of primary photoreceptor disease patients harboring other mutations. Experimental gene therapies targeting other specific mutations are in development. Other supportive measures, such as white canes, screen readers, and guide dogs help patients adapt to their visual impairments but do not address the underlying disease.
BlueRock’s Ophthalmology Program
BlueRock Therapeutics’ lead ophthalmology program, which is being developed in collaboration with Opsis Therapeutics and FUJIFILM Cellular Dynamics (FCDI), represents a promising new direction in the treatment of primary photoreceptor diseases. Through this partnership, BlueRock is working to develop iPSC-derived photoreceptor precursor cells that are transplanted via subretinal injection into the back of the eye, replacing cells that have already been lost. This type of treatment would differ from gene therapy approaches in that they are gene agnostic and can potentially address multiple indications. In addition, BlueRock has two pre-clinical programs also being developed in collaboration with Opsis and FCDI.
Primary photoreceptor diseases pose significant challenges for patients and the medical community, and the diverse genetic mutations linked to the disease and symptom variability make treatment difficult. However, BlueRock believes that innovations in cell therapy could offer new ways to transform the treatment of RP. By leveraging advanced cell therapy techniques that could potentially be used across different diseases, our team at BlueRock aims to restore vision and improve the quality of life for patients with these conditions.
